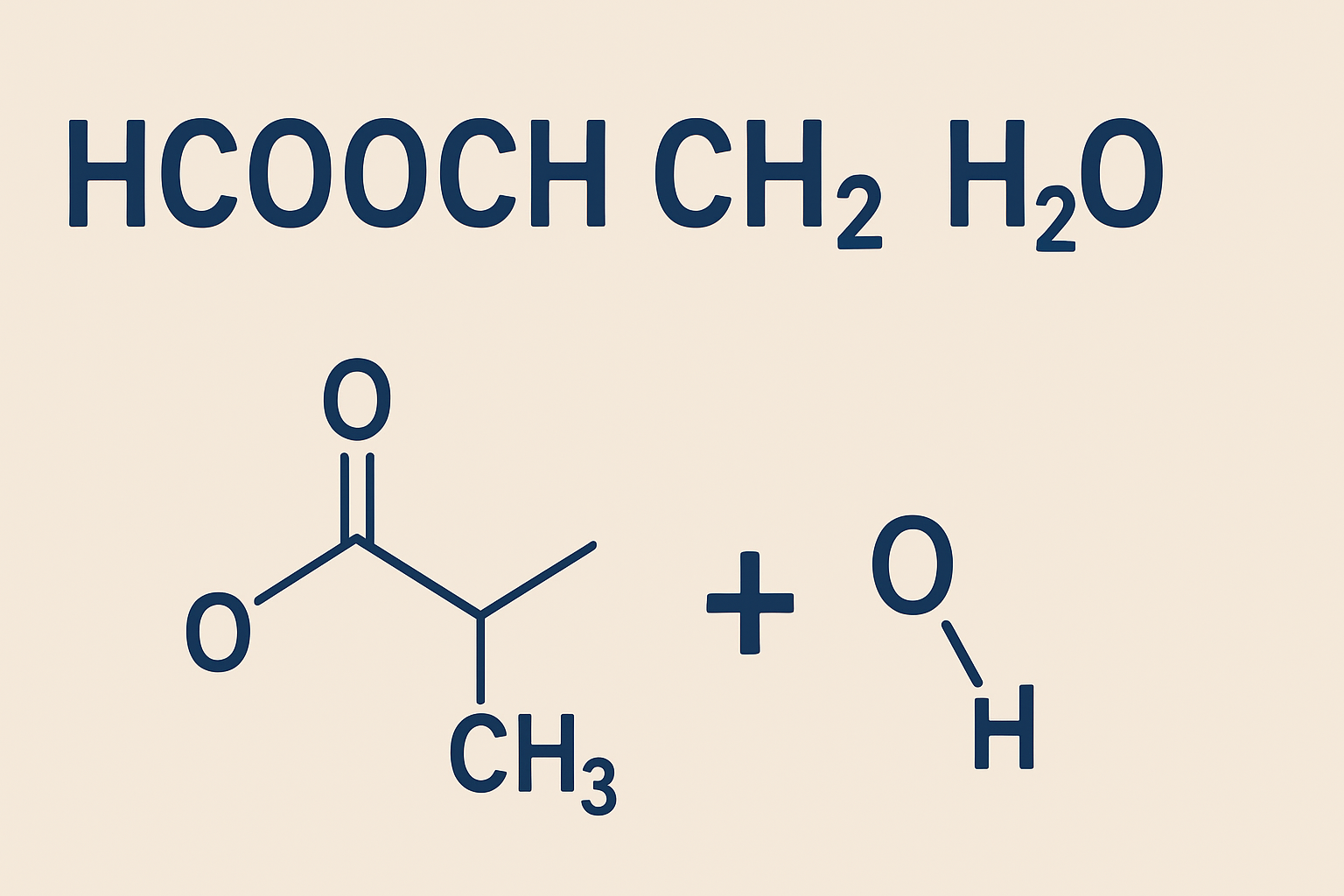Strategies for Managing Chronic Conditions in a Post-Pandemic World
October 16, 2025
Gaymetu E: Building Inclusive Gaming & Digital Identity for Marginalized Voices
October 17, 2025Explore the chemistry of hcooch ch2 h2o (methyl formate + water interactions): structure, reaction pathways, industrial applications, precautions, and FAQs explained in clear, lay-friendly terms.
also read: https://coomersparty.com/daylen-ali-carolina/
Introduction
In organic chemistry, even seemingly cryptic formulas can hide fascinating properties and practical applications. The expression hcooch ch2 h2o is one such case. At first glance, it seems like a string of fragments—“HCOOCH,” “CH₂,” and “H₂O”—but it really points us toward the chemistry of methyl formate in aqueous systems, hydrolysis reactions, and interactions involving methylene (CH₂) units in the presence of water.
In this article we will break down what hcooch ch2 h2o means, how it reacts, where it is used, how to handle it safely, and answer deeper questions beyond the core content. Our goal is to explain clearly—with no jargon overload—yet give you substantial chemical insight.
What Does “hcooch ch2 h2o” Represent?
Interpreting the Fragments
- HCOOCH suggests the core of methyl formate (which is HCOOCH₃).
- CH₂ indicates a methylene fragment, or part of a reaction intermediate.
- H₂O is water, often present in hydrolysis, hydration, or aqueous reaction media.
So, hcooch ch2 h2o is not a “single molecule” per se, but rather a shorthand that hints at the interplay between methyl formate (or a formate ester), methylene groups, and water. In practice, when someone writes hcooch ch2 h2o, they often refer to reaction systems where methyl formate encounters water, possibly with intermediate formation of CH₂ species or in condensation/hydrolysis settings.
Why This Notation Matters
This notation is useful when describing mechanisms or reaction environments. For instance:
- When methyl formate dissolves in water and hydrolyzes.
- When methylene insertions or bridging (–CH₂–) occur in aqueous media under certain catalysts.
- When you want to emphasize that the system involves these three moieties interacting.
In broad chemical literature, you’d more often see the clear formula HCOOCH₃ for methyl formate, and then write its reaction with water:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
Still, by using hcooch ch2 h2o, one draws attention to mechanistic paths where CH₂ intermediates, hydration steps, or condensation events may also arise.
Structure and Physical Properties
Structure of Methyl Formate (HCOOCH₃)
Methyl formate is the methyl ester of formic acid. Its structural formula is:
H–C(=O)–O–CH₃
This consists of:
- A formyl portion (H–C=O)
- An ester oxygen bridge (–O–)
- A methyl group (CH₃)
When present in an aqueous medium (i.e., with H₂O), hydrogen bonding, solvation, and hydration effects influence its behavior.
Molar Mass and Composition
According to molecular weight calculations, methyl formate (HCOOCH₃) has a molar mass of 59.0440 g/mol. Its elemental composition is:
- Hydrogen (H): ~5.12% by mass
- Carbon (C): ~40.68%
- Oxygen (O): ~54.19%
These figures show that oxygen contributes heavily to the mass, consistent with the ester and carbonyl functional groups.
Physical Characteristics
- State: Colorless liquid under standard conditions
- Odor: Somewhat fruity or ester-like
- Solubility: Methyl formate mixes moderately with water; in aqueous solution, interactions with H₂O molecules are important
- Boiling point / volatility: Relatively low compared to less polar liquids
- Miscibility: Partial miscibility; water will interact via hydrogen bonding with the ester group
These properties make methyl formate useful as a solvent, reagent, or intermediate in chemical syntheses where aqueous environment matters.
Reaction Behavior: Hydrolysis, Condensation, and Methylene Involvement
Hydrolysis in Water
One of the central reactions involving hcooch ch2 h2o systems is the hydrolysis of methyl formate in water:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This reaction breaks the ester bond to yield formic acid (HCOOH) and methanol (CH₃OH). It is typically catalyzed by acid or base. In acidic conditions, the protonation of the carbonyl oxygen makes the ester bond more susceptible to attack by water. In basic conditions (saponification), a nucleophilic hydroxide attacks the carbonyl, cleaving off the methanol moiety.
The presence of H₂O is essential—the reaction is driven by the water molecule’s nucleophilic attack. The interplay between the ester fragment (HCOOCH) and water underscores the notation hcooch ch2 h2o.
Involvement of CH₂ Units
Although methyl formate itself does not include a CH₂ segment, the notation CH₂ may refer to:
- Methylene intermediates: In some reaction chains, methylene (–CH₂–) fragments can appear as short-lived species, especially under radical or organometallic catalysis.
- Bridge formation: In polymerizations or condensations, CH₂ bridges may link formate or ester units.
- Functional transformation: One can imagine insertion of CH₂ groups in complex organic syntheses involving ester intermediates in aqueous media.
Therefore, hcooch ch2 h2o can also imply that methyl formate is part of a larger reaction sequence involving methylene insertion or bridging in the presence of water.
Combined Reaction Schemes
In more elaborate synthetic routes, you might see:
- Condensation of methyl formate with other substrates mediated by methylene units
- Redox processes where formic acid (from hydrolysis) acts as a hydrogen donor
- Polymer syntheses where ester fragments and CH₂ units alternate in aqueous steps
In summary, while the canonical reaction is hydrolysis, the notation hints that one might embed methyl formate reactions into broader synthetic frameworks involving CH₂ segments and aqueous environment.
Industrial and Laboratory Applications
Use as a Solvent and Intermediate
Methyl formate is often leveraged in industry as a solvent because it can dissolve oils, resins, or organic compounds to some extent, and because its volatility allows easy removal. In hcooch ch2 h2o contexts, aqueous mixtures can be tuned for reaction conditions.
In organic synthesis, methyl formate is used as a formylating agent or intermediate in multistep protocols. The presence of water (H₂O) may be intentional or as an unavoidable medium.
Catalysis, Green Chemistry, and Fuel Research
The link between formic acid and hydrogen is a current area of interest in sustainable energy. In such systems, formic acid (a hydrolysis product) can act as a hydrogen donor. The hcooch ch2 h2o framework may appear in studies where methylene bridging or other organic backbones are integrated into hydrogen storage or catalytic cycles.
Moreover, because hydrolysis of esters is mild and water-based, processes using methyl formate + water are sometimes considered “greener” compared to using strong organic solvents. The interaction motif of hcooch ch2 h2o underscores precisely that: water-mediated organic transformations.
Laboratory Techniques
When chemists work with methyl formate in aqueous conditions, typical practices include:
- Maintaining pH (often pH 2–4 for acid-catalyzed hydrolysis)
- Using catalysts (acid salts, metal complexes) to tune rate and selectivity
- Controlling temperature (elevated temperature helps hydrolysis)
- Monitoring reaction progress by techniques such as gas chromatography or NMR
If CH₂ intermediates or bridging steps are involved, more specialized catalysts (e.g. transition metal complexes) and inert atmospheres may be required.
Safety, Handling, and Environmental Notes
Safety Risks
- Flammability: Methyl formate is flammable; avoid ignition sources
- Irritation: It can irritate eyes, respiratory tract, or skin if exposure is high
- Corrosivity: Its hydrolysis product—formic acid—is corrosive; contact must be carefully managed
- Volatility: Good ventilation or fume hoods should be used when handling
Always wear gloves, goggles, and lab coat, and work in ventilated conditions.
Environmental Considerations
- Spillage or release of methyl formate or formic acid to water sources should be contained
- Waste streams should be neutralized (e.g. by buffering or base addition)
- Organic residues should be removed or disposed of per local regulations
Because the system hcooch ch2 h2o implies water-based reactions, designing closed systems or recycling water can reduce emissions and waste.
Table: Comparison of Key Entities
| Entity / Component | Role in hcooch ch2 h2o System | Key Characteristics |
|---|---|---|
| HCOOCH₃ (Methyl formate) | The ester fragment being hydrolyzed or transformed | Volatile, reactive toward water, solvent properties |
| CH₂ (Methylene fragment or bridge) | Optional intermediate or linkage unit in multi-step syntheses | Highly reactive, often transient |
| H₂O (Water) | Solvent, nucleophile, facilitator of hydrolysis | Universal solvent, participates in proton transfers |
This table helps visualize how the notation hcooch ch2 h2o brings together these three interacting roles.
Frequently Asked Questions
- Does methyl formate decompose spontaneously in pure water over time?
Under neutral pH and ambient temperature, decomposition is slow. Catalysts or acids speed it. - Can isotopic water (D₂O) be used instead of H₂O in hcooch ch2 h2o reactions?
Yes, using D₂O reveals isotope effects in hydrolysis mechanisms and helps trace pathways. - Are there enzyme catalysts that hydrolyze methyl formate in biological systems?
Some esterases might act on formate esters, though methyl formate specifically is less common biologically. - What effect does temperature have on the rate of hydrolysis in hcooch ch2 h2o?
Higher temperature accelerates hydrolysis (arrhenius behavior), but may also lead to side reactions. - Is methyl formate stable in strong acidic or alkaline aqueous solutions?
It will hydrolyze; in strong acid or base, the rate is significantly faster and may require buffer control. - How does ionic strength (salt concentration in solution) influence hcooch ch2 h2o reaction rates?
Salt can stabilize or destabilize charged intermediates, altering hydrolysis kinetics. - Can you drive the reverse reaction (esterification) in an hcooch ch2 h2o mixture?
The equilibrium lies toward hydrolysis in aqueous solution, but removing water or applying acid catalysts can favor formation of ester. - What spectroscopic methods detect changes in hcooch ch2 h2o systems?
NMR, IR (carbonyl stretching), mass spectrometry, and GC are common. - Do catalysts like metal nanoparticles help CH₂ insertion in aqueous ester systems?
Yes, certain transition metal catalysts can mediate CH₂ insertion or methylene coupling in aqueous media. - How do pressure or supercritical water conditions affect these systems?
Elevated pressure can enhance solvation, reactivity, and shift equilibria; supercritical water often accelerates transformation steps.
Conclusion
In summary, hcooch ch2 h2o is a useful shorthand to evoke the chemistry of methyl formate (HCOOCH₃) interacting with water (H₂O), potentially in contexts that involve methylene (CH₂) fragments or bridging steps. The core reaction is hydrolysis of the ester to yield formic acid and methanol, but the notation hints at more elaborate synthetic or catalytic systems where CH₂ units participate in aqueous media.
We have explored structure, physical properties, reaction behavior, industrial and lab applications, safety, and environmental aspects. The table clarifies the roles of each fragment, and the additional FAQs aim to address tangential questions that deepen your understanding.
Whether you’re a student delving into organic chemistry or a researcher exploring sustainable reaction design, grasping hcooch ch2 h2o helps you link ester chemistry, methylene chemistry, and water-mediated processes in a coherent framework.